[ad_1]
There’s a preferred false impression that cochlear implants restore pure listening to. In truth, these marvels of engineering give individuals a brand new type of “electrical listening to” that they have to learn to use.
Pure listening to outcomes from vibrations hitting tiny constructions referred to as hair cells throughout the cochlea within the interior ear. A cochlear implant bypasses the broken or dysfunctional components of the ear and makes use of electrodes to instantly stimulate the cochlear nerve, which sends indicators to the mind. When my hearing-impaired sufferers have their cochlear implants turned on for the primary time, they usually report that voices sound flat and robotic and that background noises blur collectively and drown out voices. Though customers can have many periods with technicians to “tune” and alter their implants’ settings to make sounds extra nice and useful, there’s a restrict to what could be achieved with at present’s know-how.
I’ve been an otolaryngologist for greater than twenty years. My sufferers inform me they need extra pure sound, extra enjoyment of music, and most of all, higher comprehension of speech, significantly in settings with background noise—the so-called
cocktail party problem. For 15 years, my team on the College of Göttingen, in Germany, has been collaborating with colleagues on the College of Freiburg and past to reinvent the cochlear implant in a strikingly counterintuitive means: utilizing mild.
We acknowledge that at present’s cochlear implants run up in opposition to laborious limits of engineering and human physiology. So we’re creating a brand new type of cochlear implant that makes use of mild emitters and genetically altered cells that reply to mild. Through the use of exact beams of sunshine as a substitute {of electrical} present to stimulate the cochlear nerve, we count on our optical cochlear implants to higher replicate the complete spectral nature of sounds and higher mimic pure listening to. We intention to start out scientific trials in 2026 and, if all goes properly, we may get regulatory approval for our system at first of the following decade. Then, individuals everywhere in the world may start to listen to the sunshine.
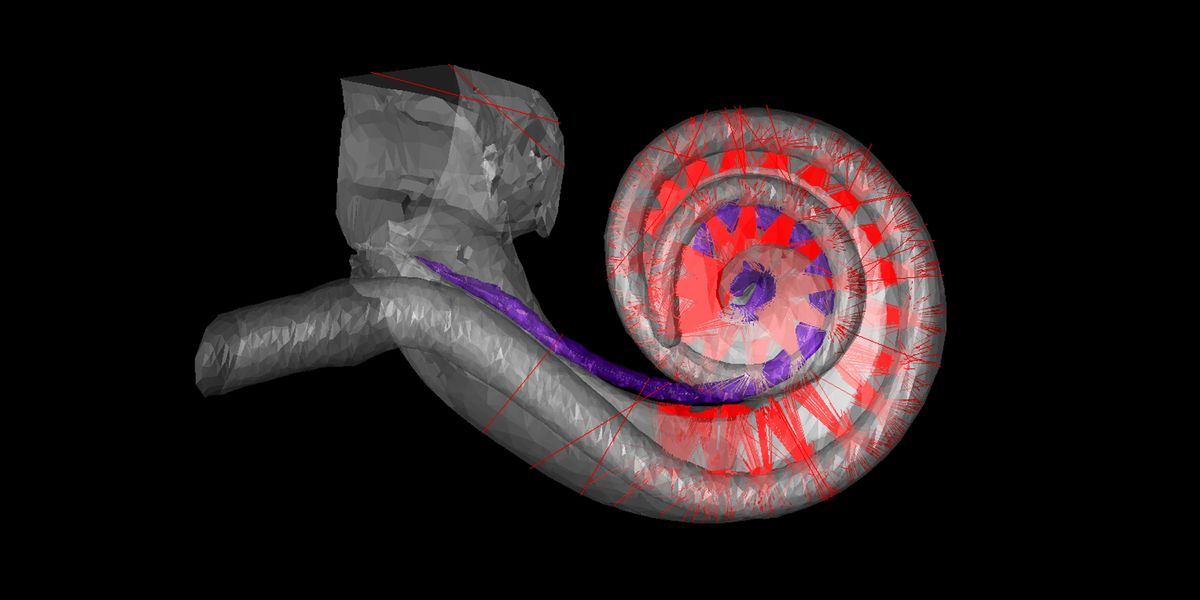
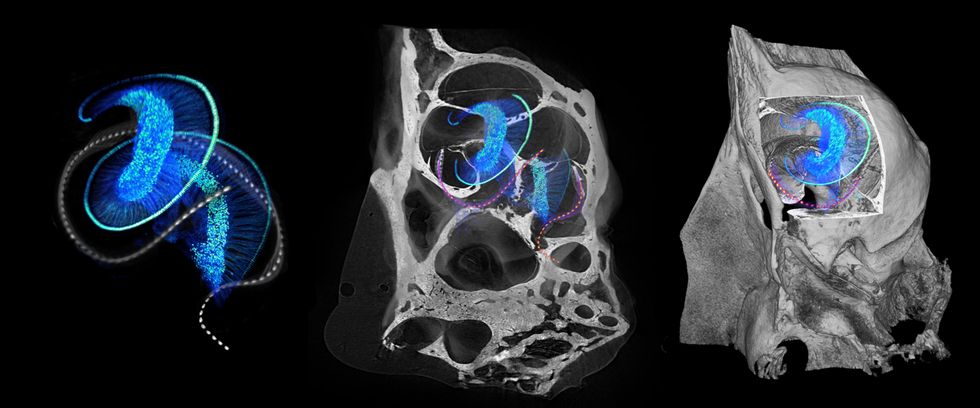 These 3D microscopic photos of mouse ear anatomy present optical implants [dotted lines] twisting by way of the intricate construction of a traditional cochlea, which incorporates hair cells; in deafness, these cells are misplaced or broken. At left, the hair cells [light blue spiral] connect with the cochlear nerve cells [blue filaments and dots]. Within the center and proper photos, the bony housing of the mouse cochlea surrounds this delicate association.Daniel Keppeler
These 3D microscopic photos of mouse ear anatomy present optical implants [dotted lines] twisting by way of the intricate construction of a traditional cochlea, which incorporates hair cells; in deafness, these cells are misplaced or broken. At left, the hair cells [light blue spiral] connect with the cochlear nerve cells [blue filaments and dots]. Within the center and proper photos, the bony housing of the mouse cochlea surrounds this delicate association.Daniel Keppeler
How cochlear implants work
Some
466 million people worldwide undergo from disabling listening to loss that requires intervention, in line with the World Well being Group. Listening to loss primarily outcomes from harm to the cochlea brought on by illness, noise, or age and, up to now, there isn’t a remedy. Listening to could be partially restored by listening to aids, which basically present an amplified model of the sound to the remaining sensory hair cells of the cochlea. Profoundly hearing-impaired individuals profit extra from cochlear implants, which, as talked about above, skip over dysfunctional or misplaced hair cells and instantly stimulate the cochlear, or auditory, nerve.
Within the 2030s, individuals everywhere in the world may start to listen to the sunshine.
At this time’s cochlear implants are essentially the most profitable neuroprosthetic thus far. The primary system was accredited by the U.S. Meals and Drug Administration within the 1980s, and
nearly 737,000 devices had been implanted globally by 2019. But they make restricted use of the neurons obtainable for sound encoding within the cochlea. To know why, you first want to grasp how pure listening to works.
In a functioning human ear, sound waves are channeled down the ear canal and set the ear drum in movement, which in flip vibrates tiny bones within the center ear. These bones switch the vibrations to the interior ear’s cochlea, a snail-shaped construction concerning the dimension of a pea. Contained in the fluid-filled cochlea, a membrane ripples in response to sound vibrations, and people ripples transfer bundles of sensory hair cells that challenge from the floor of that membrane. These actions set off the hair cells to launch neurotransmitters that trigger {an electrical} sign within the neurons of the cochlear nerve. All these electrical indicators encode the sound, and the sign travels up the nerve to the mind. No matter which sound frequency they encode, the cochlear neurons characterize sound depth by the speed and timing of their electrical indicators: The firing charge can attain just a few hundred hertz, and the timing can obtain submillisecond precision.
Hair cells in several components of the cochlea reply to completely different frequencies of sound, with these on the base of the spiral-shaped cochlea detecting high-pitched sounds of as much as about 20 kilohertz, and people on the high of the spiral detecting low-pitched sounds all the way down to about 20 Hz. This frequency map of the cochlea can also be obtainable on the degree of the neurons, which could be regarded as a spiraling array of receivers. Cochlear implants capitalize on this construction, stimulating neurons within the base of the cochlea to create the notion of a excessive pitch, and so forth.
A business cochlear implant at present has a microphone, processor, and transmitter which can be worn on the pinnacle, in addition to a receiver and electrodes which can be implanted. It sometimes has between 12 and 24 electrodes which can be inserted into the cochlea to instantly stimulate the nerve at completely different factors. However the saline fluid throughout the cochlea is conductive, so the present from every electrode spreads out and causes broad activation of neurons throughout the frequency map of the cochlea. As a result of the frequency selectivity {of electrical} stimulation is restricted, the standard of synthetic listening to is restricted, too. The pure strategy of listening to, wherein hair cells set off exact factors on the cochlear nerve, could be regarded as enjoying the piano together with your fingers; cochlear implants are extra equal to enjoying together with your fists. Even worse, this huge stimulation overlap limits the best way we are able to stimulate the auditory nerve, because it forces us to activate just one electrode at a time.
How optogenetics works
The thought for a greater means started again in 2005, after I began listening to a couple of new approach being pioneered in neuroscience referred to as
optogenetics. German researchers had been among the many first to find light-sensitive proteins in algae that regulated the stream of ions throughout a mobile membrane. Then, different analysis teams started experimenting with taking the genes that coded for such proteins and utilizing a innocent viral vector to insert them into neurons. The upshot was that shining a lightweight on these genetically altered neurons may set off them to open their voltage-gated ion channels and thus fireplace, or activate, permitting researchers to instantly management residing animals’ brains and behaviors. Since then, optogenetics has grow to be a big software in neuroscience analysis, and clinicians are experimenting with medical functions together with vision restoration and cardiac pacing.
I’ve lengthy been serious about how sound is encoded and the way this coding goes incorrect in listening to impairment. It occurred to me that stimulating the cochlear nerve with mild as a substitute of electrical energy may present rather more exact management, as a result of mild could be tightly targeted even within the cochlea’s saline surroundings.
We’re proposing a brand new kind of implanted medical system that might be paired with a brand new kind of gene remedy.
If we used optogenetics to make cochlear nerve cells mild delicate, we may then exactly hit these targets with beams of low-energy mild to supply a lot finer auditory sensations than with {the electrical} implant. We may theoretically have greater than 5 occasions as many targets spaced all through the cochlea, maybe as many as 64 or 128. Sound stimuli could possibly be electronically cut up up into many extra discrete frequency bands, giving customers a a lot richer expertise of sound. This common concept had been taken up earlier by
Claus-Peter Richter from Northwestern College, who proposed instantly stimulating the auditory nerve with high-energy infrared mild, although that idea wasn’t confirmed by different laboratories.
Our concept was thrilling, however my collaborators and I noticed a number of challenges. We had been proposing a brand new kind of implanted medical system that will be paired with a brand new kind of gene remedy, each of which should meet the very best security requirements. We’d want to find out the very best mild supply to make use of within the optogenetic system and transmit it to the correct spots within the cochlea. We needed to discover the fitting light-sensitive protein to make use of within the cochlear nerve cells, and we had to determine how finest to ship the genes that code for these proteins to the fitting components of the cochlea.
However we’ve made nice progress through the years. In 2015, the European Analysis Council gave us a vote of confidence when it
funded our “OptoHear” project, and in 2019, we spun off an organization referred to as OptoGenTech to work towards commercializing our system.
Channelrhodopsins, micro-LEDs, and fiber optics
Our early proof-of-concept experiments in mice explored each the biology and know-how at play in our mission. Discovering the fitting light-sensitive protein, or channelrhodopsin, turned out to be an extended course of. Many early efforts in optogenetics used
channelrhodopsin-2 (ChR2) that opens an ion channel in response to blue mild. We used it in a proof-of-concept experiment in mice that demonstrated that optogenetic stimulation of the auditory pathway supplied higher frequency selectivity than electrical stimulation did.
In our continued seek for the very best channelrhodopsin for our function, we tried a ChR2 variant referred to as
calcium translocating channelrhodopsin (CatCh) from the Max Planck Institute of Biophysics lab of Ernst Bamberg, one of many world pioneers of optogenetics. We delivered CatCh to the cochlear neurons of Mongolian gerbils utilizing a harmless virus as a vector. We subsequent educated the gerbils to answer an auditory stimulus, instructing them to keep away from a sure space once they heard a tone. Then we deafened the gerbils by making use of a drug that kills hair cells and inserted a tiny optical cochlear implant to stimulate the light-sensitized cochlear neurons. The deaf animals responded to this light stimulation simply as they needed to the auditory stimulus.
The optical cochlear implant will allow individuals to pick voices in a busy assembly and admire the subtleties of their favourite songs.
Nonetheless, the usage of CatCh has two issues: First, it requires blue mild, which is related to
phototoxicity. When mild, significantly high-energy blue mild, shines instantly on cells which can be sometimes in the dead of night of the physique’s inside, these cells could be broken and finally die off. The opposite downside with CatCh is that it’s gradual to reset. At physique temperature, as soon as CatCh is activated by mild, it takes a couple of dozen milliseconds to shut the channel and be prepared for the following activation. Such gradual kinetics don’t assist the exact timing of neuron activation essential to encode sound, which might require greater than 100 spikes per second. Many individuals mentioned the kinetics of channelrhodopsins made our quest not possible—that even when we gained spectral decision, we’d lose temporal decision. However we took these doubts as a powerful motivation to search for quicker channelrhodopsins, and ones that reply to pink mild.
We had been excited when a pacesetter in optogenetics,
Edward Boyden at MIT, found a faster-acting channelrhodopsin that his staff referred to as Chronos. Though it nonetheless required blue mild for activation, Chronos was the quickest channelrhodopsin thus far, taking about 3.6 milliseconds to shut at room temperature. Even higher, we discovered that it closed inside about 1 ms on the hotter temperature of the physique. Nonetheless, it took some further tips to get Chronos working within the cochlea: We had to make use of highly effective viral vectors and sure genetic sequences to enhance the supply of Chronos protein to the cell membrane of the cochlear neurons. With these tips, each single neurons and the neural inhabitants responded robustly and with good temporal precision to optical stimulation at greater charges of as much as about 250 Hz. So Chronos enabled us to elicit near-natural charges of neural firing, suggesting that we may have each frequency and time decision. However we nonetheless wanted to search out an ultrafast channelrhodopsin that operated with longer wavelength mild.
We teamed up with Bamberg to tackle the problem. The collaboration focused Chrimson, a channelrhodopsin first described by Boyden that’s finest stimulated by orange mild. The
first results of our engineering experiments with Chrimson had been quick Chrimson (f-Chrimson) and really quick Chrimson (vf-Chrimson). We had been happy to find that f-Chrimson allows cochlear neurons to respond to red light reliably as much as stimulation charges of roughly 200 Hz. Vf-Chrimson is even quicker however is much less properly expressed within the cells than f-Chrimson is; up to now, vf-Chrimson has not shown a measurable advantage over f-Chrimson with regards to high-frequency stimulation of cochlear neurons.
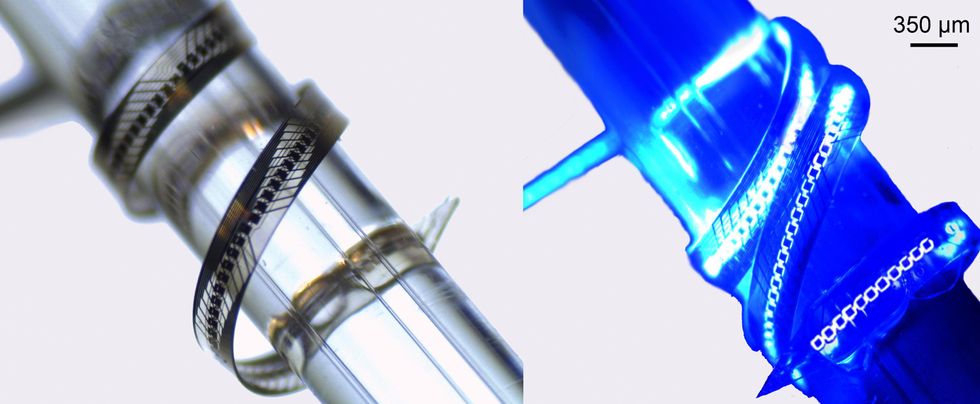 This versatile micro-LED array, fabricated on the College of Freiburg, is wrapped round a glass rod that’s 1 millimeter in diameter. The array is proven with its 144 diodes turned off [left] and working at 1 milliamp [right]. College of Freiburg/Frontiers
This versatile micro-LED array, fabricated on the College of Freiburg, is wrapped round a glass rod that’s 1 millimeter in diameter. The array is proven with its 144 diodes turned off [left] and working at 1 milliamp [right]. College of Freiburg/Frontiers
We’ve additionally been exploring our choices for the implanted mild supply that can set off the optogenetic cells. The implant have to be sufficiently small to suit into the restricted area of the cochlea, stiff sufficient for surgical insertion, but versatile sufficient to softly comply with the cochlea’s curvature. Its housing have to be biocompatible, clear, and sturdy sufficient to final for many years. My collaborators
Ulrich Schwarz and Patrick Ruther, then on the College of Freiburg, began issues off by creating the primary micro-light-emitting diodes (micro-LEDs) for optical cochlear implants.
We discovered micro-LEDs helpful as a result of they’re a really mature business know-how with good energy effectivity. We carried out
severalexperiments with microfabricated thin-film micro-LEDs and demonstrated that we may optogenetically stimulate the cochlear nerve in our focused frequency ranges. However micro-LEDs have drawbacks. For one factor, it’s tough to determine a versatile, clear, and sturdy airtight seal across the implanted micro-LEDs. Additionally, micro-LEDs with the very best effectivity emit blue mild, which brings us again to the phototoxicity downside. That is why we’re additionally one other means ahead.
As an alternative of getting the semiconductor emitter itself into the cochlea, the choice method places the sunshine supply, comparable to a laser diode, farther away in a hermetically sealed titanium housing. Optical fibers then carry the sunshine into the cochlea and to the light-sensitive neurons. The optical fibers have to be biocompatible, sturdy, and versatile sufficient to wind by way of the cochlea, which can be difficult with typical glass fibers. There’s fascinating ongoing analysis in versatile polymer fibers, which could have higher mechanical traits, however up to now, they haven’t matched glass in effectivity of sunshine propagation. The fiber-optic method may have effectivity drawbacks, as a result of we’d lose some mild when it goes from the laser diode to the fiber, when it travels down the fiber, and when it goes from the fiber to the cochlea. However the method appears promising, because it ensures that the optoelectronic parts could possibly be safely sealed up and would possible make for a simple insertion of the versatile waveguide array.
 One other design chance for optical cochlear implants is to make use of laser diodes as a lightweight supply and pair them with optical fibers fabricated from a versatile polymer. The laser diode could possibly be safely encapsulated exterior the cochlea, which would scale back considerations about warmth, whereas polymer waveguide arrays [left and right images] would curl into the cochlea to ship the sunshine to the cells.OptoGenTech
One other design chance for optical cochlear implants is to make use of laser diodes as a lightweight supply and pair them with optical fibers fabricated from a versatile polymer. The laser diode could possibly be safely encapsulated exterior the cochlea, which would scale back considerations about warmth, whereas polymer waveguide arrays [left and right images] would curl into the cochlea to ship the sunshine to the cells.OptoGenTech
The highway to scientific trials
As we contemplate assembling these parts right into a business medical system, we first search for components of current cochlear implants that we are able to undertake. The audio processors that work with at present’s cochlear implants could be tailored to our function; we’ll simply want to separate up the sign into extra channels with smaller frequency ranges. The exterior transmitter and implanted receiver additionally could possibly be much like current applied sciences, which can make our regulatory pathway that a lot simpler. However the really novel components of our system—the optical stimulator and the gene remedy to ship the channelrhodopsins to the cochlea—would require an excellent quantity of scrutiny.
Cochlear implant surgical procedure is sort of mature and sometimes takes solely a few hours at most. To maintain issues easy, we need to preserve our process as shut as doable to current surgical procedures. However the important thing a part of the surgical procedure might be fairly completely different: As an alternative of inserting electrodes into the cochlea, surgeons will first administer viral vectors to ship the genes for the channelrhodopsin to the cochlear nerve cells, after which implant the sunshine emitter into the cochlea.
Since optogenetic therapies are simply starting to be examined in scientific trials, there’s nonetheless some uncertainty about how finest to make the approach work in people. We’re nonetheless occupied with get the viral vector to ship the required genes to the proper neurons within the cochlea. The viral vector we’ve utilized in experiments to this point, an
adeno-associated virus, is a innocent virus that has already been accredited to be used in several gene therapies, and we’re utilizing some genetic tips and native administration to focus on cochlear neurons particularly. We’ve already begun gathering knowledge concerning the stability of the optogenetically altered cells and whether or not they’ll want repeated injections of the channelrhodopsin genes to remain conscious of mild.
Our roadmap to scientific trials may be very bold. We’re working now to finalize and freeze the design of the system, and we’ve got ongoing preclinical research in animals to test for phototoxicity and show the efficacy of the essential concept. We intention to start our first-in-human examine in 2026, wherein we’ll discover the most secure dose for the gene remedy. We hope to launch a big section Three scientific trial in 2028 to gather knowledge that we’ll use in submitting the system for regulatory approval, which we may win within the early 2030s.
We foresee a future wherein beams of sunshine can carry wealthy soundscapes to individuals with profound listening to loss or deafness. We hope that the optical cochlear implant will allow them to pick voices in a busy assembly, admire the subtleties of their favourite songs, and take within the full spectrum of sound—from trilling birdsongs to booming bass notes. We predict this know-how has the potential to light up their auditory worlds.
From Your Website Articles
Associated Articles Across the Internet
Source link